…[T]here are several PI3K signaling pathway targeting drugs in clinical development for use against ovarian cancer and solid tumors, including GDC-0941, BEZ235, SF1126, XL-147, XL-765, BGT226, and PX-866. The results of two recent medical studies suggest that the use of PI3K-targeted therapies may offer an effective therapeutic approach for patients with advanced-stage and recurrent ovarian cancer, including a generally chemotherapy-resistant histological subtype of epithelial ovarian cancer known as “ovarian clear cell cancer” (OCCC). The targeting of the PI3K pathway in endometrial, ovarian, and breast cancer is also being investigated by a Stand Up To Cancer “Dream Team.” …
PI3K Cellular Signaling Pathway — An Overview

PI3K/AKT cellular signaling pathway (Photo: Cell Signaling Technology(R))
In 2004 and 2005, multiple researchers identified mutations in the PIK3CA gene with respect to multiple cancers.[1] The PIK3CA gene encodes the PI3K catalytic subunit p110α. PI3K (phosphoinositide 3- kinase) proteins have been identified in crucial signaling pathways of ovarian cancer cells. PI3Ks are also part of the PI3K-AKT-mTOR signaling pathway which promotes cellular glucose metabolism, proliferation, growth, survival, and invasion and metastasis in many cancers. PIK3CA gene mutations can increase PI3K signaling, thereby activating the PI3K-AKT-mTOR pathway within cancer cells.
As of this writing, there are several PI3K signaling pathway targeting drugs in clinical development for use against ovarian cancer and solid tumors, including GDC-0941, BEZ235, SF1126, XL-147, XL-765, BGT226, and PX-866. [2] The results of two recent medical studies suggest that the use of PI3K-targeted therapies may offer an effective therapeutic approach for patients with advanced-stage and recurrent ovarian cancer, including a generally chemotherapy-resistant histological subtype of epithelial ovarian cancer known as “ovarian clear cell cancer” (OCCC). The targeting of the PI3K pathway in endometrial, ovarian, and breast cancer is also being investigated by a Stand Up To Cancer “Dream Team.”
Frequent Mutation of PIK3CA Gene In Recurrent & Advanced Clear Cell Ovarian Cancer
OCCC is one of the five major subtypes of epithelial ovarian cancer. OCCC accounts for only 4% to 12% of epithelial ovarian cancer in Western countries and, for unknown reasons, it comprises more than 20% of such cancers in Japan [3,4,5]. OCCC possesses unique clinical features such as a high incidence of stage I disease, a large pelvic mass, an increased incidence of venous thromboembolic complications, and hypercalcemia. It is frequently associated with endometriosis. Compared to serous ovarian cancer, OCCC is relatively resistant to conventional platinum and taxane-based chemotherapy. For these reasons, new effective therapies are desperately needed for OCCC.
Researchers from Johns Hopkins and the University of California, Los Angeles (UCLA) analyzed 97 OCCC tumors for genetic sequence mutations in KRAS (v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog), BRAF (v-raf murine sarcoma viral oncogene homolog B1), PIK3CA (phosphoinositide-3-kinase, catalytic, alpha polypeptide), TP53 (tumor protein p53), PTEN (phosphatase and tensin homolog), and CTNNB1 (Catenin, Beta-1) as these mutations frequently occur in other major types of ovarian cancers.[6] The samples tested included the following:
- 18 OCCCs for which affinity-purified tumor cells from fresh specimens were available;
- 10 OCCC tumor cell lines.
Upon test completion, the researchers discovered that sequence mutations of PIK3CA, TP53, KRAS, PTEN, CTNNB1, and BRAF occurred in 33%, 15%, 7%, 5%, 3%, and 1% of OCCC cases, respectively.
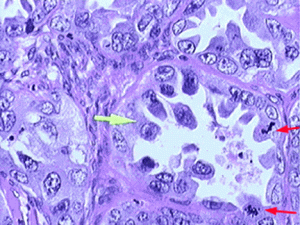
Clear cell carcinoma of the ovary (Photo: Geneva Foundation For Medical Education & Research)
The sequence analysis of the 18 affinity purified OCCC tumors and the 10 OCCC cell lines showed a PIK3CA mutation frequency of 46%. Based upon these findings the researchers concluded that the use of PIK3CA-targeting drugs may offer a more effective therapeutic approach compared with current chemotherapeutic agents for patients with advanced-stage and recurrent OCCC. As noted above, there are several PI3K-targeting drugs in clinical development for use against ovarian cancer and solid tumors.[2]
Notably, one of the researchers involved with this OCCC study is Dennis J. Slamon, M.D., Ph.D. Dr. Slamon serves as the Director of Clinical/Translational Research, and as Director of the Revlon/UCLA Women’s Cancer Research Program at the Jonsson Comprehensive Cancer Center. Dr. Slamon is also a professor of medicine, chief of the Division of Hematology/Oncology and Executive Vice Chair of Research for UCLA’s Department of Medicine. Dr. Slamon is a co-discoverer of the breast cancer drug Herceptin®. Herceptin is a monoclonal antibody targeted therapy used against HER-2 breast cancer, an aggressive breast cancer subtype that affects 20% to 30% of women with the disease. Herceptin’s development was based, in part, upon the unique genetic profile of HER-2 breast cancer as compared to other forms of breast cancer. Herceptin® revolutionized the treatment of HER-2 postive breast cancer and is recognized worldwide as the standard of care for that subtype of breast cancer. The approach taken by Johns Hopkins and UCLA researchers in this study — the identification of a subtype within a specific form of cancer that may be susceptible to a targeted therapy — bears a striking similarity to the overarching approach taken in the development of Herceptin®.
Ovarian Cancer & Other Solid Tumors With PIK3CA Gene Mutations Respond To PI3K-AKT-mTOR Pathway Inhibitors In Phase I Clinical Testing.
Testing patients with cancer for PIK3CA gene mutations is feasible and may allow targeted treatment of the PI3K-AKT-mTOR cellular signaling pathway, according to the results of a University of Texas, M.D. Anderson Cancer Center study presented on November 17, 2009 at the 2009 AACR (American Association for Cancer Research)-NCI (National Cancer Institute)-EORTC (European Organization For Research & Treatment of Cancer) International Conference on Molecular Targets and Cancer Therapeutics.[7]

mTOR cellular signaling pathway (Photo: Cell Signaling Technology(R))
Filip Janku, M.D., Ph.D, a clinical research fellow with the M.D. Anderson Cancer Center’s department of investigational cancer therapeutics, and colleagues conducted a mutational analysis of exon 9 and exon 20 of the PI3KCA gene using DNA from the tumors of patients referred to targeted therapy clinical trials. Patients with PIK3CA mutations were preferably treated whenever possible with regimens utilizing PI3K-AKT-mTOR signaling pathway inhibitors.
As part of this study 117 tumor samples were analyzed. PIK3CA mutations were detected in 14 (12%) patients. In tumor types with more than 5 patients tested, PIK3CA mutations were identified in endometrial cancer (43%, 3 out of 7 patients), ovarian cancer (22%, 5 out of 23 patients), squamous head and neck cancer (14%, 1 out of 7 patients), breast cancer (18%, 2 out of 11 patients), and colon cancer (15%, 2 out of 13 patients). No mutations were identified in patients with melanoma or cervical cancer.
Of the 14 patients found to possess PIK3CA mutations, 10 were treated based upon a clinical trial protocol that included a drug targeting the PI3K-AKT-mTOR pathway. A partial response to treatment was experienced by 4 (40%) patients. Although the total number of patients is small, there were 2 (67%) patient responses in 3 endometrial cancer cases, 1 (25%) patient response in 4 ovarian cancer cases, 1 (100%) patient response in 1 breast cancer, and no patient response in 1 colorectal cancer case. Although the total number of study patients is small, the researchers conclude that the response rate appears high (40%) in tumors with PIK3CA mutations treated with PI3K-AKT-mTOR pathway inhibitors.
“The implications of this study are twofold,” said Dr. Janku. “We demonstrated that PIK3CA testing is feasible and may contribute to the decision-making process when offering a patient a clinical trial. Although this study suffers from low numbers, the response rate observed in patients treated with inhibitors of PI3K/AKT/mTOR pathway based on their mutational status was well above what we usually see in phase-1 clinical trials.” “These results are intriguing but at this point should be interpreted with caution,” said Janku. “The promising response rate needs to be confirmed in larger groups of patients. We expect to learn more as this project continues to offer PIK3CA screening to patients considering a phase-1 clinical trial.”
Stand Up 2 Cancer Dream Team: Targeting the PI3K Pathway in Women’s Cancers
 The potential importance of the PI3K pathway in the treatment of ovarian cancer is emphasized by the two medical studies above. This issue is also receiving considerable attention from one of the Stand Up 2 Cancer (SU2C) “Dream Teams,” which is going to evalute the potential for targeting the PI3K pathway in women’s cancer. SU2C assigned $15 million of cancer research funding to this critical issue. The scientists involved in this SU2C Dream Team are the pioneers who discovered the PI3K pathway and validated its role in human cancers, and they will focus on breast, ovarian and endometrial cancers, all of which possess the PI3K mutation.
The potential importance of the PI3K pathway in the treatment of ovarian cancer is emphasized by the two medical studies above. This issue is also receiving considerable attention from one of the Stand Up 2 Cancer (SU2C) “Dream Teams,” which is going to evalute the potential for targeting the PI3K pathway in women’s cancer. SU2C assigned $15 million of cancer research funding to this critical issue. The scientists involved in this SU2C Dream Team are the pioneers who discovered the PI3K pathway and validated its role in human cancers, and they will focus on breast, ovarian and endometrial cancers, all of which possess the PI3K mutation.
The leader and co-leaders of the PI3K pathway SU2C team are set forth below.
Leader:
Lewis C. Cantley, Ph.D., Director, Cancer Center at Beth Israel Deaconess Medical Center.
Co-Leaders:
Charles L. Sawyers, M.D., Director, Human Oncology and Pathogenesis Program, Memorial Sloan-Kettering Cancer Center.
Gordon B. Mills, M.D., Ph.D., Chair, Department of Systems Biology, University of Texas, M.D. Anderson Cancer Center.
The specific SU2C Dream Team research goal with respect to targeting the PI3K pathway in women’s cancers is stated as follows:
The PI3K pathway is mutated in more cancer patients than any other, and these mutations are the most frequent events in women’s cancers, making it an attractive molecular target for agents that inhibit these genetic aberrations. If successful, this project will allow clinicians to use biomarkers and imaging techniques to predict which patients will benefit from PI3K pathway inhibitors and lead to the development of therapeutic combinations that will hit multiple targets in the complex pathways that contribute to cancer cell growth. This work will help assure that these therapies are given to patients who will benefit from them, and it will also increase the overall pace of clinical trials targeting PI3K inhibitors.
Based upon the two studies discussed, and the creation and funding of the SU2C Dream Team for the purpose of targeting the PI3K pathway in women’s cancer, the future holds great promise in the battle against ovarian cancer (including OCCC). It is our hope that more clinical study investigators will offer PI3K pathway mutation screening to all ovarian cancer patient volunteers. Libby’s H*O*P*E*™ will continue to monitor the clinical development of PI3K pathway inhibitors, and make our readers aware of all future developments.
________________________________
References:
1/Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008 Sep 18;27(41):5497-510. PubMed PMID: 18794884
Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006 Jan;18(1):77-82. PubMed PMID: 16357568.
Levine DA, Bogomolniy F, Yee CJ, et. al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005 Apr 15;11(8):2875-8. PubMed PMID: 15837735.
Samuels Y, Wang Z, Bardelli A, et. al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. Epub 2004 Mar 11. PubMed PMID: 15016963.
2/For open ovarian cancer clinical trials using a PI3K-targeted therapy; CLICK HERE; For open solid tumor clinical trials using a PI3K-targeted therapy, CLICK HERE.
3/ Itamochi H, Kigawa J & Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Can Sci 2008 Apr;99(4):653-658. [PDF Document]
4/Schwartz DR, Kardia SL, Shedden KA, et. al. Gene Expression in Ovarian Cancer Reflects Both Morphology and Biological Behavior, Distinguishing Clear Cell from Other Poor-Prognosis Ovarian Carcinomas. Can Res 2002 Aug; 62, 4722-4729.
5/Sugiyama T & Fujiwara K. Clear Cell Tumors of the Ovary – Rare Subtype of Ovarian Cancer, Gynecologic Cancer, American Society of Clinical Oncology (ASCO) Educational Book, 2007 ASCO Annual Meeting, June 2, 2007 (Microsoft Powerpoint presentation).
6/Kuo KT, Mao TL, Jones S, et. al. Frequent Activating Mutations of PIK3CA in Ovarian Clear Cell Carcinoma. Am J Pathol. 2009 Apr 6. [Epub ahead of print]
7/Janku F, Garrido-Laguna I, Hong D.S. PIK3CA mutations in patients with advanced cancers treated in phase I clinical trials, Abstract #B134, Molecular Classification of Tumors, Poster Session B, 2009 AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference. [PDF Document].













