British Columbian researchers discover that approximately one-half of clear-cell ovarian cancers and one-third of endometrioid ovarian cancers possess ARID1A gene mutations, as reported today in the New England Journal of Medicine.
British Columbian researchers discover that approximately one-half of ovarian clear-cell cancers (OCCC) and one-third of endometrioid ovarian cancers possess ARID1A (AT-rich interactive domain 1A (SWI-like)) gene mutations, as reported today in the New England Journal of Medicine (NEJM). The research paper is entitled ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas, and represents, in large part, the collaborative work of Drs. David Hunstman and Marco Marra.

Dr. David Huntsman, Co-Founder & Acting Director, Ovarian Cancer Research Program of British Columbia

Dr. Marco Marra, Director, Michael Smith Genome Sciences Centre, British Columbia Cancer Agency
David Huntsman, M.D., FRCPC, FCCMG, is a world-renowned genetic pathologist, and the Co-Founder and Acting Director of the Ovarian Cancer Research Program of British Columbia (OvCaRe). He also heads the Centre for Translational and Applied Genomics, located in the British Columbia (BC) Cancer Agency’s Vancouver Centre. Dr. Huntsman is the Co-Director of the Genetic Pathology Evaluation Centre, Vancouver General Hospital, and the Associate Director of the Hereditary Cancer Program, BC Cancer Agency. He is involved in a broad range of translational cancer research and, as the OvCaRe team leader, has studied the genetic and molecular structure of ovarian cancer for many years. In June 2009, the NEJM published one of Dr. Huntsman’s most recent groundbreaking discoveries: the identification of mutations in the FOXL2 gene as the molecular basis of adult granulosa cell ovarian cancer tumors.
Marco Marra, Ph.D. is the Director of the BC Cancer Agency’s Michael Smith* Genome Sciences Centre (GSC) , one of eight BC Cancer Agency specialty laboratories. Dr. Marra is internationally recognized as a preeminent leader in the field of genetics. His leadership has helped transform the GSC into one of the world’s most advanced and productive centers for development and application of genomics, bioinformatics and related technologies. The work of the GSC , along with collaborations involving the BC Cancer Agency and other local, national and international researchers and organizations, have led to several major scientific breakthroughs over the past decade.
*Dr. Michael Smith won the 1993 Nobel Prize in chemistry for his development of oligonucleotide-based site-directed mutagenesis, a technique which allows the DNA sequence of any gene to be altered in a designated manner. His technique created an groundbreaking method for studying complex protein functions, the basis underlying a protein’s three-dimensional structure, and a protein’s interaction with other molecules inside the cell.
Tackling Ovarian Cancer, “One Subtype At a Time”
In December 2008, the OvCaRe team announced an important discovery about the genetics of ovarian cancer – that instead of being one single disease, it is made up of a spectrum of distinct diseases. “Until now,” says OvCaRe team leader David Huntsman, “ovarian cancer has been treated as a single disease both in the cancer clinic and the research lab.” This may help explain why there have been many fewer advances in ovarian cancer research and treatment than for other cancer types.
On the heels of this important finding, Huntsman says his team decided to tackle ovarian cancers “one subtype at a time.” For its first target, the team chose granulosa cell ovarian tumors, which account for five percent of ovarian tumors and have no known drug treatments. Working with research colleagues at the GSC, Huntsman’s team used the latest genomic sequencing equipment to decipher the genetic code of this ovarian cancer subtype.
“[T]en years ago, ovarian cancer appeared to be an unsolvable problem—the liberating moment came when we established that ovarian cancer is actually a number of distinct diseases … We tailor our research approach to each subtype with the hope of developing effective treatments specific to each disease.”
— Dr. David Huntsman, Co-Founder & Acting Director, Ovarian Cancer Research Program of British Columbia.
The genomic sequencing study results were illuminating, says Huntsman, as the research team was able to identify “a single ‘spelling mistake’ in this tumor’s DNA.” Still, Huntsman is buoyed by the promise of this research and its potential to save lives. “We’ve had dozens of letters and emails from women around the world with granulosa cell tumors, who’ve written to thank us saying this discovery has given them hope they never thought they would have. Reading these letters has been both incredibly humbling and inspiring for our team.” Libby’s H*O*P*E*™ reported Dr. Huntsman’s critical ovarian cancer discovery on June 10, 2009.
The OvCaRe team’s research findings have already been used to advance the care of BC patient Barbara Johns, a fourth grade teacher whose granulosa cell tumor was surgically removed in February 2009. “This could lead to new non-surgical treatment options for patients with this type of cancer,” says Johns, who was the first patient to benefit from the new diagnostic test. “It’s definitely a step in the right direction.”
Listen to a brief audio excerpt taken from an interview with Dr. David Huntsman, in which he explains why this is an exciting time for ovarian cancer research.
The Ovarian Cancer Research Program of British Columbia

Select NEJM Article Authors (left to right): Drs. Sohrab Shah, David Huntsman, Dianne Miller, C. Blake Gilks
OvCaRe, a multi-institutional and multi-disciplinary ovarian cancer research group, was developed as a collaboration between the BC Cancer Agency, the Vancouver Coastal Health Research Institute, and the University of British Columbia. The OvCaRe program includes clinicians and research scientists from Vancouver General Hospital (VGH) and the BC Cancer Agency, who specialize in gynecology, pathology, and medical oncology. As noted above, Dr. Huntsman leads the OvCaRe team as its Co-Founder and Acting Director.
A team approach has ensured the building of translational research platforms, accessible to all OvCaRe team members regardless of institutional affiliation or medical/scientific discipline. The OvCaRe program research platforms include a gynecologic cancer tumor bank, the Cheryl Brown Ovarian Cancer Outcomes Unit, a tissue microarray core facility for biomarker studies, a xenograft core facility for testing experimental therapeutics, and a genomics informatics core facility. OvCaRe is developing two additional core facilities to improve knowledge dissemination and clinical trials capacity.
Although OvCaRe was formed less than ten years ago, the team has been recognized for several groundbreaking medical and scientific discoveries related to the understanding and management of ovarian cancer. The significant discoveries reported within the past two years are listed below.
- Proved that various subtypes of ovarian ovarian are distinct diseases, and reported that potential treatment advances depend on both clinically managing and researching these subtypes as separate entities (2008)( PMID: 19053170).
- Identified mutations in the FOXL2 gene as the molecular basis of adult granulosa cell ovarian cancer tumors using next generation sequencing – the first clinically relevant discovery made with this new technology (2009)(PMID: 19516027).
- Discovered that women with earlier stage ovarian clear-cell cancer may benefit from lower abdominal radiation therapy (2010)(PMID: 20693298).
In many cases, these contributions have already led to changes in clinical practice in British Columbia. The international reputation of Vancouver’s OvCaRe team ensures that the positive impact of these changes is felt immediately throughout British Columbia, while also being emulated in other jurisdictions worldwide. These contributions were made possible due to the population-based cancer system in British Columbia and strong support from the BC Cancer Foundation and the Vancouver General Hospital (VGH) & University of British Columbia (UBC) Hospital Foundation.
Background: Ovarian Clear-Cell Cancer
Ovarian cancer ranks as the 5th deadliest cancer among U.S. women.[1] There are four general subtypes of epithelial ovarian cancer — serous, clear-cell, endometrioid, and mucinous.[2] High-grade serous ovarian cancer is the most common and represent approximately 70% of all cases of epithelial ovarian cancer in North America. [3]
The OCCC subtype represents 12 percent of ovarian cancers in North America; however, it represents up to 20 percent of ovarian cancers diagnosed in Japan and other East Asian countries. [3,4] OCCC possesses unique clinical features such as a high incidence of stage I disease, a large pelvic mass, an increased incidence of vascular thromboembolic complications, and hypercalcemia. [4-6] Both OCCC and endometrioid ovarian cancer are frequently associated with endometriosis. [4-6] The genetic events associated with the transformation of endometriosis into ovarian clear-cell cancer and endometrioid cancer are unknown.
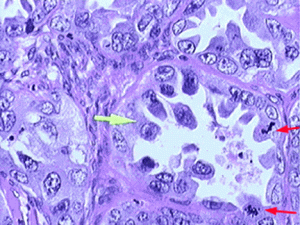
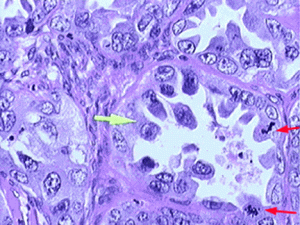
Clear cell carcinoma of the ovary
OCCC does not respond well to the standard platinum and taxane-based ovarian cancer chemotherapy: response rates are 15 per cent compared to 80 per cent for the most common type of ovarian cancer, high-grade serous ovarian cancer. [4-6] However, the exact mechanisms underlying OCCC’s resistance to chemotherapy is not fully understood. Although several mechanisms involved in drug resistance exist in OCCC, including decreased drug accumulation, increased drug detoxification, increased DNA repair activity [4-6], and low proliferation activity[4]; no particular chemoresistance system has been identified. Due to the general chemoresistant nature of OCCC, it is generally stated that the prognosis for advanced-stage or recurrent OCCC is poor. [3, 7-8] The prognosis for OCCC that is diagnosed in Stage I, and treated by complete cytoreduction that results in little or no residual disease, is usually good. [8-10]
Although OCCC is the second leading cause of death from ovarian cancer, it is relatively understudied by the medical and research community. Despite this fact, there have been a few important studies involving this subtype of ovarian cancer.
Various researchers have long noted that OCCC has a distinct genetic profile, as compared to other types of epithelial ovarian cancer.[6, 11-14] Gene expression profiling can serve as a powerful tool to determine biological relationships, if any, between tumors. In fact, National Cancer Institute (NCI) and Memorial Sloan-Kettering Cancer Center (MSKCC) researchers observed that clear-cell cancers share similarity in gene expression profiles, regardless of the human organ of origin (including kidney), and could not be statistically distinguished from one another. [13] The researchers found that the same was not true for the non-OCCC forms of epithelial ovarian cancer. Several investigators have made similar observations. [14-16] It is important to note, however, that there are significant genetic differences between OCCC and renal clear-cell cancer (RCCC). For example, abnormalities of the VHL (Von Hippel-Lindau)/HIF1-α (Hypoxia-inducible factor 1-alpha) pathway have been identified in the majority of RCCC cases, but not in OCCC cases. [17, 18]
The basic finding that clear-cell tumors show remarkably similar gene expression patterns regardless of their organ of origin is provocative. This NCI/MSKCC study finding raises the question of whether therapies used to treat RCCC would be effective against OCCC. Targeted-therapies such as VEGFR inhibitors (e.g., sunitinib (Sutent®)), PDGFR inhibitors (e.g., sorafenib (Nexavar®)), m-TOR inhibitors (e.g., temsirolimus (Torisel®) & everolimus (Afinitor®)), and anti-angiogenesis drugs (e.g., bevacizumab (Avastin®)) are used to treat RCCC. Notably, Fox Chase Cancer Center researchers performed preclinical testing of everolimus on ovarian cancer cell lines and xenografted mice and observed significant anti-tumor activity. [19, 20] The Division of Clinical Gynecologic Oncology at the Massachusetts General Hospital also observed the anti-tumor effect of sunitinib in one refractory OCCC patient that recurred after nine years and four prior treatment lines. [21] Japanese researchers have also highlighted this potential approach to fighting OCCC. [22-25]
All of the above-mentioned drugs used to treat RCCC are currently being tested in ovarian cancer and solid tumor clinical studies. Accordingly, these drugs are generally available to advanced-stage and recurrent OCCC patients who do not respond to prior taxane/platinum therapy and other standard lines of treatment, assuming such patients satisfy all clinical study enrollment criteria. [26-30]
In a 2009 study conducted by researchers at Johns Hopkins and University of California, Los Angeles (UCLA), it was discovered that approximately one-third of OCCCs contained PIK3CA (phosphoinositide-3-kinase, catalytic, alpha polypeptide) gene mutations. [31] Testing patients with cancer for PIK3CA gene mutations may be feasible and allow targeted treatment of the PI3K-AKT–mTOR cellular signaling pathway, according to the results of a University of Texas, M.D. Anderson Cancer Center study presented at the 2009 AACR (American Association for Cancer Research)-NCI-EORTC (European Organization For Research & Treatment of Cancer) International Conference on Molecular Targets and Cancer Therapeutics. [31] The M.D. Anderson study results may carry great significance in the future because there are several PI3K signaling pathway targeting drugs in clinical development for use against ovarian cancer and solid tumors. [32]
Also in 2009, researchers affiliated with UCLA, the Mayo Clinic, and Harvard Medical School announced that they established a biological rationale to support the clinical study of the U.S. Food & Drug Administration (FDA)-approved leukemia drug dasatinib (Sprycel®), either alone or in combination with chemotherapy, in patients with ovarian cancer (including OCCC). [33]
In August 2010, Dr. Ken Swenerton, a senior OvCaRe team member and co-leader of OvCaRe’s Cheryl Brown Ovarian Cancer Outcomes Unit, reported provocative findings relating to the use of adjuvant radiotherapy to fight OCCC. [34] Dr. Swenerton is also a co-chair of the NCI Gynecologic Cancer Steering Committee (GCSC) Ovarian Cancer Task Force. The NCI GCSC determines all phase III clinical trials for gynecologic cancers in the U.S. and other jurisdictions. The population-based, retrospective study conducted by OvCaRe reported that a 40 percent decrease in disease specific mortality was associated with adjuvant radiotherapy administered to women with stage I (other than grade 1 tumors), II, & III clear-cell, endometrioid, and mucinous ovarian cancers, who possessed no residual (macroscopic) disease following complete cytoreductive surgery. Although the study dataset was too small to discriminate effects among the clear-cell, endometrioid and mucinous ovarian cancer histologies, the overall results highlight the curative potential of adjuvant radiotherapy in select non-serous ovarian cancer patients. Moreover, there is limited scientific and anecdotal evidence set forth in past studies that supports the select use of radiotherapy against OCCC. [35-38]
BRCA 1 (BReast CAncer gene 1) & BRCA 2 (BReast CAncer gene 2) mutations increase a woman’s lifetime risk of breast and ovarian cancer. [39] In at least one small study, BRCA2 germline (inherited) and somatic (non-inherited) gene mutations were identified in 46 percent of the OCCC samples tested. [40] This provocative study brings into question the potential use of PARP (Poly (ADP-ribose) polymerase) inhibitors against OCCC in select patients. [41] PARP inhibitors have shown effectiveness against germline BRCA gene mutated ovarian cancers, [42, 43] and may be effective against somatic BRCA gene mutated ovarian cancers. [44, 45]
International researchers continue to identify theoretical therapeutic drug targets for OCCC. These targets include: IGF2BP3 (insulin-like growth factor 2 mRNA-binding protein 3) [46], HNF-1beta (hepatocyte nuclear factor-1beta) [47], annexin A4 [48], GPC3(Glypican-3) [49], osteopontin [50], sFRP5 (secreted frizzled-related protein 5) [51], VCAN (versican) [52], transcription factor POU6F1 (POU class 6 homeobox 1) [53], and microRNA mir-100 [54].
Although researchers have identified that OCCC is distinct from high-grade serous carcinoma, OCCC-specific biomarkers and treatments have not been broadly adopted. Despite the theoretical approaches and study results highlighted above, there are no definitive (i.e., clinically-proven) anti-cancer agents for OCCC, and without understanding the molecular basis of this ovarian cancer subtype in much greater detail, the development of more targeted therapies is unlikely.
NEJM ARID1A Study Methodology
The OvCaRe team research consisted of four major analyses as described below.
- RNA Sequencing of OCCC Tumor Samples and Cell Line (Discovery Cohort)
By way of background, DNA (deoxyribonucleic acid) is the genetic material that contains the instructions used in the development and functioning of our cells. DNA is generally stored in the nucleus of our cells. The primary purpose of DNA molecules is the long-term storage of information. Often compared to a recipe or a code, DNA is a set of blueprints that contains the instructions our cells require to construct other cell components, such as proteins and RNA (ribonucleic acid) molecules. The DNA segments that carry this genetic information are called genes.
RNA is the genetic material that transcribes (i.e., copies) DNA instructions and translates them into proteins. It is RNA’s job to transport the genetic information out of the cell’s nucleus and use it as instructions for building proteins. The so-called “transcriptome” consists of all RNA molecules within our cells, including messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA). The sequence of RNA mirrors the sequence of the DNA from which it was transcribed or copied. Consequently, by analyzing the entire collection of RNAs (i.e., the transcriptome) in a cell, researchers can determine when and where each gene is turned on or off in our cells and tissues. Unlike DNA, the transcriptome can vary with external environmental conditions. Because it includes all mRNA transcripts in the cell, the transcriptome reflects the genes that are being actively expressed at any given time.
A gene is essentially a sentence made up of the bases A (adenine), T (thymine), G (guanine), and C (cytosine) that describes how to make a protein. Any change in the sequence of bases — and therefore in the protein instructions — is a mutation. Just like changing a letter in a sentence can change the sentence’s meaning, a mutation can change the instruction contained in the gene. Any changes to those instructions can alter the gene’s meaning and change the protein that is made, or how or when a cell makes that protein.
Gene mutations can (i) result in a protein that cannot carry out its normal function in the cell, (ii) prevent the protein from being made at all, or (iii) cause too much or too little of a normal protein to be made.
The first study analysis involved the RNA sequencing of 18 patient OCCC tumors and 1 OCCC cell line. The primary purpose of this step was to discover any prevalent genetic mutations within the sample tested. Specifically, the research team sequenced the whole transcriptomes of the OCCC tumors and the single OCCC cell line and discovered a variety of somatic (non-inherited) mutations in the ARID1A gene. The researchers also found mutations in CTNNB1(catenin beta-1 gene), KRAS (v-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue gene), and PIK3CA (phosphoinositide-3-kinase, catalytic, alpha polypeptide gene).
ARID1A encodes the BAF250a protein, a key component of the SWI-GNF chromatin remodeling complex which regulates many cellular processes, including development, differentiation, proliferation, DNA repair, and tumor suppression. [55] The BAF250a protein encoded by ARID1A is believed to confer specificity in regulation of gene expression.
To date, mutations or other aberrations in ARID1A have not been identified in ovarian cancer, but have been identified in breast and lung cancer cell lines. [56] Other researchers have suggested that ARID1A is a tumor-suppressor gene. [56]
- DNA Sequencing of OCCC Tumor Samples and Cell Lines (Discovery Cohort + Mutation Validation Cohort)
The finding of multiple types of mutations in a single gene, ARID1A, within the discovery cohort, led researchers to perform a mutation validation analysis. The researchers only conducted analyses with respect to ARID1A, because it was already known that mutations in CTNNB1, KRAS, and PIK3CA are recurrent in ovarian cancer. [31, 57]
This step of the research involved DNA sequencing of 210 samples of various subtypes of ovarian cancer and one OCCC cell line, along with the 18 OCCC tumor samples and one OCCC cell line used in the discovery cohort. Upon completion of the DNA sequencing, the researchers identified ARID1A mutations in 55 of 119 (46%) OCCCs, 10 of 33 (30%) endometrioid cancers, and none of the 76 high-grade serous cancers. Also, the researchers found primarly somatic (non-inherited) truncating mutations.
Based on the second study analysis, the researchers report that the presence of ARID1A mutations are strongly associated with OCCCs and endometrioid cancers. These two subtypes of ovarian cancer, as noted above, are associated with endometriosis.
- Testing For BAF250a Protein Expression
In the third study analysis, the researchers used immunohistochemical analysis (IHC) to measure BAF250a protein expression in 450 ovarian cancers.
The first round of IHC testing involved 182 ovarian cancers which were available from the discovery cohorts and the mutation-validation cohorts: 73 OCCCs, 33 endometrioid cancers, and 76 high-grade serous ovarian cancers. The goal of the first IHC analysis was to compare the loss of BAF250a protein expression in OCCCs and endometrioid cancers, with and without ARID1A mutations. Upon completion, the researchers identified loss of BAF250a protein expression in 27 of 37 (73%) OCCCs, and 5 of 10 (50%) endometrioid cancers, which possessed ARID1A mutations. In contrast, loss of BAF250a protein expression was identified in only 4 of 36 (11%) OCCCs, and 2 of 23 (9%) endometrioid cancers, which did not possess ARID1A mutations. Thus, the loss of BAF250a protein expression was much greater in OCCCs and endometrioid cancers with ARID1A mutations.
The goal of the second IHC analysis was to compare loss of BAF250a protein expression among all OCCCs, endometrioid cancers, and high-grade serous cancers. The researchers identified loss of BAF250a protein expression in 31 of 73 (42%) OCCCs, and 7 of 33 (21%) endometrioid cancers, as compared to 1 of 76 (1%) high-grade serous cancers. Thus, the loss of BAF250a protein expression was much greater in the OCCCs and endometrioid cancers, as compared to high-grade serous cancers, regardless of ARID1A mutation status.
The second round of IHC testing measured loss of BAF250a protein expression within the IHC validation cohort. This analysis revealed that 55 of 132 (42%) OCCCs, 39 of 125 (31%) endometrioid cancers, and 12 of 198 (6%) high-grade serous cancers, lost BAF250a protein expression.
By the end of IHC testing, the researchers established that the loss of BAF250a protein expression was consistently more common in OCCCs and endometrioid cancers than in high-grade serous cancers, when assessed in the discovery and mutation-validation cohorts, and again in the IHC cohort.
The researchers also reported that no significant associations with loss of BAF250a protein expression were noted on the basis of age at disease presentation, disease stage, or disease-specific survival within any of the ovarian cancer subtypes.
- Analysis of ARID1A Gene Mutations & BAF250a Protein Expression In Continguous Atypical Endometriosis
The fourth study analysis evaluated samples taken from two OCCC patients who had ARID1A mutations and contiguous atypical endometriosis. In both instances, the patient sample included the primary OCCC tumor, clones derived from contiguous atypical endometriosis, and clones derived from a distant endometriotic lesion.
In the first patient, ARID1A mutations were identified in the OCCC tumor, and 17 of 42 clones derived from contiguous atypical endometriosis, but in none of the 52 clones derived from a distant endometriotic lesion. The samples taken from this patient’s OCCC tumor and atypical endometriosis revealed loss of BAF250a protein expression; however, expression was maintained in the distant endometriotic lesion. HNF-1beta was expressed in the OCCC tumor, but not in the contiguous atypical or distant endometriosis. Estrogen receptor expression tested positive in both the contiguous atypical and distant endometriosis, but not in the OCCC tumor.
In the second patient, ARID1A mutations and a CTNNB1 mutation were identified in the OCCC tumor and contiguous atypical endometriosis, but not in a distant endometriotic lesion.
Results Summary
Based on the foregoing discussion, the major OvCaRe study findings are summarized below.
- 46% of patients with OCCC and 30% of those with endometrioid cancers had somatic (non-inherited) truncating or missense mutation in the ARID1A gene.
- No ARID1A mutations were identified in the 76 high-grade serous cancers analyzed.
- Loss of BAF250a protein expression was identified in 36% of OCCCs and endometrioid cancers, but in only 1% of high-grade serous cancers.
- Loss of BAF250a protein expression was seen in 73% and 50% of OCCCs and endometrioid cancers with an ARID1A mutation, respectively, and in only 11% and 9% of samples without ARID1A mutations, respectively.
- The majority of cancers possessing somatic ARID1A mutations and loss of BAF250a expression appear to have a normal (also known as “wild-type”) allele present.
- DNA and RNA sequencing data reveals that the ratio of abnormal (mutant) to normal (wild-type) alleles at both the DNA and RNA levels is consistent, thereby suggesting that epigenetic silencing is not a significant factor.
- In two patients, ARID1A mutations and loss of BAF250a protein expression were identified in the OCCC tumor and contiguous atypical endometriosis, but not in distant endometriotic lesions.
Conclusions
The researchers note in the study that ARID1A is located at chromosome 1p36.11. Although this fact carries little meaning for a layperson, the researchers explain that this chromosomal region is commonly deleted in tumors, and that such deletions could contain tumor-suppressor genes. Based upon the totality of the data, the OvCaRe team believes that ARID1A is a tumor-suppressor gene which is frequently disrupted in OCCCs and endometrioid cancers. Although a bit speculative due to small sample size, the researchers also believe that because ARID1A mutation and loss of BAF250a protein expression were identified in precancerous endometriotic lesions, such events represent a transformation of endometriosis into cancer.
“The finding that ARID1A is the most frequently mutated gene described thus far in endometrioid and clear cell ovarian cancers represents a major scientific breakthrough. This discovery also sheds light on how endometriosis predisposes to the development of these cancers. The novel insights provided by this work have the exciting potential to facilitate advances in early diagnosis, treatment and prevention of endometrioid and clear cell cancers, which account for over 20 per cent of ovarian cancer cases.”
—Dr. Andrew Berchuck, Director, Division of Gynecologic Oncology, Duke University Medical Center
Inaugural Ovarian Clear-Cell Carcinoma Symposium

International Clear-Cell Carcinoma of the Ovary Symposium (June 24, 2010)
On June 24, 2010, a group of preeminent clinicians and cancer research scientists from around the world gathered for the Clear Cell Carcinoma of the Ovary Symposium (the Symposium), which was held at the University of British Columbia. To my knowledge, the Symposium is the first global scientific meeting dedicated to a specific subtype of ovarian cancer, namely OCCC.
At the invitation of Dr. David Huntsman, the founder of the Symposium, I had the distinct pleasure and honor of attending this prestigious and informative meeting as an observer. Dr. Huntsman was aware that my 26-year old cousin, Libby, died from OCCC, and he thought that the Libby’s H*O*P*E*™ community would benefit from the information presented at the Symposium.
The stated goal of the Symposium was to empower the international clinical and research community interested in OCCC, and allow that community to focus on the major barriers to improving OCCC outcomes. Moreover, the Symposium speakers and attendees were charged with presenting unpublished data and providing provocative OCCC questions for group discussion. The countries represented at that Symposium included Australia, Canada, Italy, Japan, the United Kingdom, and the U.S.
The 1-day event was presented through three major sessions. The first session addressed issues that challenge the clinical dogma relating to OCCC, and covered topic areas such as epidemiology, surgery, pathology, systemic oncology, and radiation oncology. The second session addressed OCCC molecular pathology and genomics. The third session addressed global OCCC translational research and covered topic areas including OCCC outcomes from conventional clinical trials, current OCCC clinical trials, and novel approaches to OCCC treatment and the testing of new agents.
The international Symposium presenters, included the following individuals:
- David Bowtell, Group Leader, Cancer Genetics & Genomics Research Laboratory, Peter MacCallum Cancer Centre; Program Head, Cancer Genetics & Genomics, Peter MacCallum Cancer Centre, Melbourne (Australia).
- Michael A. Quinn, MB ChB Glas. MGO Melb. MRCP FRCOG FRANZCOG CGO, Director of Oncology/Dysplasia, Royal Women’s Hospital, Melbourne, Australia; Professor, Department of Obstetrics and Gynecology, University of Melbourne; Chair, National Cancer Control Initiative; Chair, Education Committee, International Gynecological Cancer Society; Chair, Ovarian Cancer Research Group, Cancer Council; Member, National Expert Advisory Group on Ovarian Cancer. (Australia)
- C. Blake Gilks, M.D., FRCPC, Co-Founder, Ovarian Cancer Research Program of BC; Professor & Acting Head, Department of Pathology and Laboratory Medicine, University of British Columbia; Head of Anatomic Pathology, Vancouver General Hospital; Member, Vancouver Coastal Health Research Institute; Co-Founder & Co-Director, Genetic Pathology Evaluation Centre, Vancouver General Hospital. (Canada)
- Paul Hoskins, MA, M.B. B. CHIR, MRCP., FRCPC, Clinical Professor, University of British Columbia. (Canada)
- David Huntsman, M.D., FRCPC, FCCMG, Co-Founder & Acting Director, Ovarian Cancer Research Program of British Columbia; Director, Centre for Translational and Applied Genomics, BC Cancer Agency; Co-Director, Genetic Pathology Evaluation Centre, Vancouver General Hospital; Associate Director, Hereditary Cancer Program, BC Cancer Agency. (Canada)
- Helen MacKay, M.D., Staff Physician, Division of Medical Oncology and Hematology, Princess Margaret Hospital; Assistant Professor, University of Toronto; Member: (i) ICON 7 Translational Committee (representing NCIC CTG), (ii) Study Committee of the TFRI Ovarian Cancer Biomarker Program, (iii) Gynecologic Cancer Steering Committee Cervical Cancer Task Force: Intergroup/NCI/National Institutes of Health, (iv) Cervix Working Group (NCIC CTG), (v) Gynecologic Disease Site Group (Cancer Care Ontario), and (vi) the GOC CPD Committee. (Canada)
- Amit M. Oza, Bsc, MBBS, M.D., FRCPC, FRCP, Senior Staff Physician & Professor of Medicine, Princess Margaret Hospital, University of Toronto; Clinical Studies Resource Centre Member, Ontario Cancer Institute. (Canada)
- Ken Swenerton, M.D., Co-Leader, Cheryl Brown Ovarian Cancer Outcomes Unit, Ovarian Cancer Research Program of BC; Clinical Professor, Medical Oncology, University of British Columbia; Department of Pathology, Vancouver Coastal Health Research Institute; Genetic Pathology Evaluation Centre,Vancouver General Hospital; Co-Chair, NCI Gynecologic Cancer Steering Committee Ovarian Cancer Task Force. (Canada).
- Anna Tinker, M.D., FRCPC, Clinical Assistant Professor, University of British Columbia, Department of Medicine; Medical Oncologist, Oncology, British Columbia Cancer Agency (Canada).
- Gillian Thomas, M.D., FRCPC, Professor, Department of Radiation Oncology & Obstetrics and Gynecology, University of Toronto; Radiation Oncologist, Odette Cancer Centre; Co-Chair, NCI Gynecologic Cancer Steering Committee; Member, ACRIN Gynecologic Committee; Member, Cervix Committee and Executive Committee, Gynecologic Cancer Intergroup (GCIG); Member, Cervix Committee – Gynecologic Oncology Group (GOG); Associate Editor, International Journal of Gynecologic Cancer. (Canada)
- Aikou Okamoto, M.D., Department of Obstetrics & Gynecology, Jikei University School of Medicine, Tokyo (Japan).
- Ian McNeish, MA, Ph.D., MRCP, MRC, Senior Clinical Fellow, Professor of Gynecological Oncology & Honorary Consultant in Medical Oncology, Deputy Director of the Barts Experimental Cancer Medicine Centre, Institute of Cancer, Barts and the London School of Medicine. (United Kingdom) (See Libby’s H*O*P*E*™, April 7, 2009)
- Michael J. Birrer, M.D., Ph.D., Director of GYN/Medical Oncology at the Massachusetts General Hospital Cancer Center; Professor, Department of Medicine, Harvard Medical School; Co-Chair, NCI Gynecologic Cancer Steering Committee; formerly, Chief of the Molecular Mechanisms Section, Cell and Cancer Biology Branch, NCI Center for Cancer Research; formerly official representative from NCI Center for Cancer Research to the Gynecological Cancer Steering Committee. (United States)(See Libby’s H*O*P*E*™, December 8, 2009)
OvCaRe Ovarian Clear-Cell Carcinoma Research Initiative
As noted above, OCCC has been identified as distinct subtype of ovarian cancer. OCCC-specific biomarkers or treatments have not been broadly adopted. Moreover, there are currently no clinically proven anti-cancer agents for OCCCs. For this reason, the OvCaRe team and other BC Cancer Agency scientists, have initiated a pioneering OCCC research initiative that consists of six separate, but interrelated projects.
The project will begin with the most fundamental research, the large scale sequencing of RNA and DNA derived from OCCC tumors. In the second, concurrent project, the vast quantities of genome sequence data will be transformed into usable knowledge that will be evaluated for clinical relevance by local and international experts. Identifying and validating novel biomarkers from the data obtained will be the focus of the third project, and the fourth project will permit scientists to specifically target those cellular biochemical signaling pathways that are considered to be useful tools for future drug development. The development and testing of the therapeutic targets and new drugs or new combinations of drugs in animal and human testing will complete this initiative.
The OvCaRe and the BC Cancer Agency scientists have a unique opportunity to completely reshape the scientific and medical understanding of OCCC and impact the way patients with this rare form of cancer are treated. The strength of their research initiative is based on linking the clinical research resources developed through OvCaRe with the genomic sequencing capacity of the BC Cancer Agency’s Genome Sciences Centre, and the drug development capacity of the Centre for Drug Research and Development and the NanoMedicine Research Group.
“This pioneering discovery by Dr. Huntsman and his dedicated ovarian cancer research team will allow the international research community to take the genomic ‘high ground’ in the battle against these formidable subtypes of epithelial ovarian cancer. The Ovarian Cancer Research Program of BC’s reported findings represent a critical first step towards development of one or more personalized targeted therapies to combat these lethal forms of ovarian cancer.”
—Paul Cacciatore, Founder, Libby’s H*O*P*E*™
The impact of this research may not be experienced by women diagnosed with OCCC today, but this foundational research must begin immediately so as to impact outcomes in the years to come. Ably led by Dr. David Huntsman, this team of dedicated individuals represents a depth and breadth of medical and scientific expertise not often found in a single geographic location.
The hope is that through the identification of therapeutic targets for OCCC, this team will yield a powerful “superstar” drug such as Herceptin (used successfully for HER-2 positive breast cancer) or Gleevec (used successfully for chronic myelogenous leukemia (CML)). These drugs are examples of therapeutics that were created based on a direct match of an identified genetic target to the therapeutic solution.
This project is of utmost importance as it will define the unique aspects of OCCC and lead to the development of more effective therapies for women diagnosed with this rare subtype of ovarian cancer.
Special Acknowledgments
First and foremost, I want to thank Dr. Huntsman for his intelligence, creative vision and compassion, which he utilizes to great effect each day, in conducting scientific research designed to ultimately benefit all women with OCCC. I also want to thank Dr. Huntsman for the generous invitation to attend the OCCC Symposium in June. It was a privilege and honor to attend and listen to international OCCC experts discuss and debate the merits of various approaches to beating this subtype of epithelial ovarian cancer. In sum, Dr. Huntsman has been extremely generous to me with respect to his time and expertise during my recent trip to Vancouver and throughout my preparation of this article.
Prior to today’s ARID1A gene mutation discovery announcement, women with OCCC did not have a “voice” in the cancer research scientific community. Dr. Huntsman has not only given these women a voice, he has given them hope for the future. As the late Christopher Reeve said: “Once you choose hope, anything is possible.”
I also want to thank the OvCaRe team members and BC Cancer Agency scientists that I met in Vancouver during my June trip, including Ken Swenerton, M.D., Sohrab Shah, Ph.D., Dianne Miller, M.D., Sam Aparicio, Ph.D., and Blake Gilks, M.D., for taking the time to answer all of my novice questions with a great understanding and passion.
Simply stated, this article would not have been possible without the substantial assistance provided to me by Sharon Kennedy, a Senior Director of Development with the BC Cancer Foundation. Sharon exemplifies the “heart and soul” behind the BC Cancer Foundation’s philanthropic activities.
Last, but certainly not least, I want to thank Mr. Douglas Gray, a highly successful entrepreneur and attorney, for introducing me to the BC scientific cancer research community. Doug is a tireless supporter of all women with OCCC, through his compassion, caring, and philanthropic generosity.
The Talmud says: “And whoever saves a life, it is considered as if he saved an entire world.” Doug Gray is in the business of saving women’s lives.
_________________________________
References:
1/Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin 2010 July 7 (Epub ahead of print).
2/Cellular Classification of Ovarian Epithelial Cancer, Ovarian Epithelial Cancer Treatment (PDQ®)(Health Professional Version), National Cancer Institute, July 9, 2010.
3/Köbel M, Kalloger SE, Huntsman DG, et al. Differences in tumor type in lowstage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 2010;29:203-11.
4/Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci 2008;99:653-8.
5/Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, et. al. Gene Expression in Ovarian Cancer Reflects Both Morphology and Biological Behavior, Distinguishing Clear Cell from Other Poor-Prognosis Ovarian Carcinomas. Can Res 2002 Aug; 62, 4722-4729.
6/Sugiyama T & Fujiwara K. Clear Cell Tumors of the Ovary – Rare Subtype of Ovarian Cancer, Gynecologic Cancer, ASCO Educational Book, 2007 ASCO Annual Meeting, June 2, 2007 (Microsoft Powerpoint presentation).
7/Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008 Jun;109(3):370-6. [Epub 2008 Apr 18].
8/Ma SK, Zhang HT, Wu LY, Liu LY. Prognostic analysis of 88 patients with ovarian clear cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2007 Oct;29(10):784-8.
9/Takano M, Sasaki N, Kita T, Kudoh K, Fujii K, Yoshikawa T et. al. Survival analysis of ovarian clear cell carcinoma confined to the ovary with or without comprehensive surgical staging; Oncol Rep. 2008 May;19(5):1259-64.
10/Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H et. al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006 May 22;94(10):1369-74.
11/Sugiyama T, Kumagai S, & Hatayama S. Treatments of epithelial ovarian cancer by histologic subtype. Gan To Kagaku Ryoho. 2009 Feb;36(2):187-92.
12/Pectasides D, Pectasides E, Psyrri A, Economopoulos T. Treatment Issues in Clear Cell Carcinoma of the Ovary: A Different Entity?. Oncologist. 2006 Nov-Dec;11(10):1089-94.
13/Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ et. al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer; Clin Cancer Res. 2005 Sep 15;11(18):6422-30.
14/Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, et. al. Gene Expression Patterns in Ovarian Carcinomas; Mol. Bio. Cell 2003 Dec.; 14(11):4376-4386.
15/Tan DS, Kaye S. Ovarian clear cell adenocarcinoma: a continuing enigma. J Clin Pathol. 2007 Apr;60(4):355-60. Epub 2006 Oct 3.
16/ Dent J, Hall GD, Wilkinson N, Perren TJ, Richmond I, Markham AF, et. al. Cytogenetic alterations in ovarian clear cell carcinoma detected by comparative genomic hybridisation. Br J Cancer. 2003 May 19;88(10):1578-83.
17/Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist 2007;12:1404-1415.
18/Köbel M, Xu H, Bourne PA, Spaulding BO, Shih IM; Mao TL et. al. IGF2BP3 (IMP3) Expression Is a Marker of Unfavorable Prognosis in Ovarian Carcinoma of Clear Cell Subtype. Modern Pathology. 2009;22(3):469-475. [Epub 2009 Jan 9].
19/Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et. al. RAD001[everolimus] inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin. Cancer. Res. 2007 Jul; 13, 4261-4270.
20/Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et. al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007 Mar 15;67(6):2408-13.
21/Rauh-Hain JA, Penson RT. Potential benefit of Sunitinib in recurrent and refractory ovarian clear cell adenocarcinoma. Int J Gynecol Cancer. 2008 Sep-Oct;18(5):934-6. Epub 2007 Dec 13.
22/Yoshida S, Furukawa N, Haruta S, et. al. Theoretical model of treatment strategies for clear cell carcinoma of the ovary: focus on perspectives. Cancer Treat Rev. 2009 Nov;35(7):608-15. Epub 2009 Aug 8. Review.
23/Mabuchi S, Kawase C, Altomare DA, et. al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res. 2009 Sep 1;15(17):5404-13. Epub 2009 Aug 18.
24/Miyazawa M, Yasuda M, Fujita M, et. al. Therapeutic strategy targeting the mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma. Pathol Int. 2009 Jan;59(1):19-27.
25/Mabuchi S, Kawase C, Altomare DA, et. al. Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary. Mol Cancer Ther. 2010 Aug;9(8):2411-22. Epub 2010 Jul 27.
26/For open ovarian cancer clinical trials using sunitinib, CLICK HERE; For open solid tumor clinical trials using sunitinib, CLICK HERE.
27/For open ovarian cancer clinical trials using sorafenib CLICK HERE; For open solid tumor clinical trials using sorafenib, CLICK HERE.
28/For open ovarian cancer clinical trials using temsirolimus, CLICK HERE; For open solid tumor clinical trials using temsirolimus, CLICK HERE.
29/For open ovarian cancer clinical trials using everolimus, CLICK HERE; For open solid tumor clinical trials using everolimus, CLICK HERE.
30/For open ovarian cancer clinical trials using bevacizumab, CLICK HERE; For open solid tumor clinical trials using bevacizumab, CLICK HERE.
31/PI3K Pathway: A Potential Ovarian Cancer Therapeutic Target?, by Paul Cacciatore, Libby’s H*O*P*E*™, November 30, 2009.
32/For open ovarian cancer clinical trials using a phosphoinositide 3′-kinase (PI3K)-targeted therapy; CLICK HERE; For open solid tumor clinical trials using a phosphoinositide 3′-kinase (PI3K)-targeted therapy, CLICK HERE.
33/UCLA Researchers Significantly Inhibit Growth of Ovarian Cancer Cell Lines With FDA-Approved Leukemia Drug Dasatinib (Sprycel®),by Paul Cacciatore, Libby’s H*O*P*E*™, November 30, 2009.
34/Swenerton KD, Santos JL, Gilks CB, et. al. Histotype predicts the curative potential of radiotherapy: the example of ovarian cancers. Ann Oncol. 2010 Aug 6. [Epub ahead of print]
35/Nagai Y, Inamine M, Hirakawa M, et. al. Postoperative whole abdominal radiotherapy in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2007 Dec;107(3):469-73. Epub 2007 Aug 31.
36/Skirnisdottir I, Nordqvist S, Sorbe B. Is adjuvant radiotherapy in early stages (FIGO I-II) of epithelial ovarian cancer a treatment of the past? Oncol Rep. 2005 Aug;14(2):521-9. PubMed PMID: 16012740.
37/Takai N, Utsunomiya H, Kawano Y, et. al. Complete response to radiation therapy in a patient with chemotherapy-resistant ovarian clear cell adenocarcinoma. Arch Gynecol Obstet. 2002 Dec;267(2):98-100.
38/Suzuki M, Saga Y, Tsukagoshi S, et. al. Recurrent ovarian clear cell carcinoma: complete remission after radiation in combination with hyperthermia; a case study and in vitro study. Cancer Biother Radiopharm. 2000 Dec;15(6):625-8.
39/BRCA1 and BRCA2: Cancer Risk and Genetic Testing, National Cancer Institute Fact Sheet, Cancer Topic, National Cancer Institute, May 29, 2009.
40/Goodheart MJ, Rose SL, Hattermann-Zogg M, et. al. BRCA2 alteration is important in clear cell carcinoma of the ovary. Clin Genet. 2009 Aug;76(2):161-7. Epub 2009 Jul 28.
41/For open ovarian cancer clinical trials using PARP inhibitors, CLICK HERE; For open solid tumor clinical trials using PARP inhibitors, CLICK HERE.
42/Audeh MW, Carmichael J, Penson RT, et. al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010 Jul 24;376(9737):245-51. Epub 2010 Jul 6.
43/PARP Inhibitor Olaparib Benefits Women With Inherited Ovarian Cancer Based Upon Platinum Drug Sensitivity, by Paul Cacciatore, Libby’s H*O*P*E*™, April 23, 2010.
44/Konstantinopoulos PA, Spentzos D, Karlan BY, et. al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010 Aug 1;28(22):3555-61. Epub 2010 Jun 14.
45/Bast RC Jr, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol. 2010 Aug 1;28(22):3545-8. Epub 2010 Jun 14.
46/Köbel M, Xu H, Bourne PA, et. al. IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis in ovarian carcinoma of clear cell subtype. Mod Pathol. 2009 Mar;22(3):469-75. Epub 2009 Jan 9.
47/Köbel M, Kalloger SE, Carrick J, Huntsman D, et. al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009 Jan;33(1):14-21.
48/Kim A, Serada S, Enomoto T, Naka T. Targeting annexin A4 to counteract chemoresistance in clear cell carcinoma of the ovary. Expert Opin Ther Targets. 2010 Sep;14(9):963-71.
49/Maeda D, Ota S, Takazawa Y, et. al. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009 Jun;22(6):824-32. Epub 2009 Mar 27.
50/Matsuura M, Suzuki T, Saito T. Osteopontin is a new target molecule for ovarian clear cell carcinoma therapy. Cancer Sci. 2010 Aug;101(8):1828-33. Epub 2010 May 12.
51/Ho CM, Lai HC, Huang SH, et. al. Promoter methylation of sFRP5 in patients with ovarian clear cell adenocarcinoma. Eur J Clin Invest. 2010 Apr;40(4):310-8.
52/Yamaguchi K, Mandai M, Oura T, et. al. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010 Mar 25;29(12):1741-52. Epub 2010 Jan 11.
53/Yoshioka N, Suzuki N, Uekawa A, et. al. POU6F1 is the transcription factor that might be involved in cell proliferation of clear cell adenocarcinoma of the ovary. Hum Cell. 2009 Nov;22(4):94-100.
54/Nagaraja AK, Creighton CJ, Yu Z, et. al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol. 2010 Feb;24(2):447-63. Epub 2010 Jan 15.
55/Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene 2009;28:1653-68.
56/Huang J, Zhao YL, Li Y, et. al. Genomic and functional evidence for an ARID1A tumor suppressor role. Genes Chromosomes Cancer 2007;46:745-50.
57/Largest Study Matching Genomes To Potential Anticancer Treatments Releases Initial Results, by Paul Cacciatore, Libby’s H*O*P*E*™, August 3, 2010.
_________________________________
Sources:
_________________________________
Genetics 101
The information hyperlinked above was obtained from GeneticHealth & the BC Cancer Agency’s Michael Smith Genome Sciences Centre.
About David Huntsman, M.D., FRCPC, FCCMG
David Huntsman, M.D., FRCPC, FCCMG, is a world-renowned genetic pathologist, and the Co-Founder and Director of the Ovarian Cancer Research Program of British Columbia(OvCaRe). He also heads the Centre for Translational and Applied Genomics, located in the British Columbia (BC) Cancer Agency’s Vancouver Centre. Dr. Huntsman is also the Co-Director of the Genetic Pathology Evaluation Centre, Vancouver General Hospital, and the Associate Director of the Hereditary Cancer Program, BC Cancer Agency. He is involved in a broad range of translational cancer research and, as the OvCaRe team leader, has studied the genetic and molecular structure of ovarian cancer for many years.
His recent retrospective assessment of 21 candidate tissue-based biomarkers implicated that ovarian cancer subtypes are different diseases, contributing to the view that contemplation of disease subtype is crucial to the study of ovarian cancer. To ultimately beat ovarian cancer, Huntsman and his dedicated OvCaRe team believe that ovarian cancer must be genetically tackled “one subtype at a time.” In June 2009, the NEJM published one of Dr. Huntsman’s most recent groundbreaking discoveries: the identification of mutations in the FOXL2 gene as the molecular basis of adult granulosa cell ovarian cancer tumors. As of today, Dr. Huntsman and his OvCaRe team can add to their groundbreaking discoveries, the identification of frequent ARID1A gene mutations in endometriosis-associated ovarian cancers (i.e., the clear-cell and endometrioid ovarian cancer subtypes).
About Marco Marra, Ph.D.
Marco Marra, Ph.D. is the Director of the BC Cancer Agency’s Michael Smith Genome Sciences Centre (GSC), one of eight BC Cancer Agency specialty laboratories. Dr. Marra is internationally recognized as a preeminent leader in the field of genetics. His leadership has helped transform the GSC into one of the world’s most advanced and productive centers for development and application of genomics, bioinformatics and related technologies.
The work of the GSC , along with collaborations involving the BC Cancer Agency and other local, national and international researchers and organizations, have led to several major scientific breakthroughs over the past decade. These breakthroughs include the rapid genome sequencing of the SARS Coronavirus, and the sequencing and genome analysis of the avian flu (H7N3).
About the Ovarian Cancer Research Program of British Columbia (OvCaRe)
The Ovarian Cancer Research Program of BC was formed in late 2000 when a group of Vancouver-based physicians and scientists joined with the common vision of enhancing ovarian cancer research in British Columbia and the explicit goal of improving outcomes for ovarian cancer patients. OvCaRe was developed as a collaboration between the BC Cancer Agency, the Vancouver Coastal Health Research Institute, and the University of British Columbia. The OvCaRe program includes clinicians and research scientists from the Vancouver General Hospital (VGH) and the British Columbia (BC) Cancer Agency, who specialize in gynecology, pathology, and medical oncology.
OvCaRe is currently focused on three major goals.
1. To improve ovarian cancer survival through early detection of disease. OvCaRe researchers are working to identify proteins that are produced in the early stages of ovarian cancer. Detection of these proteins can then be developed into diagnostic tests to allow for earlier diagnosis of ovarian cancer.
2. To develop new therapies for ovarian cancer treatment. This is being achieved through research aimed at identifying the cause of ovarian cancer at the cellular level and then directly and specifically targeting that defect. OvCaRe is using a similar strategy to develop treatments to prevent ovarian cancer recurrence.
3. To develop individualized ovarian cancer treatments. Ovarian cancer can be subdivided into several groups based on their pathological appearance, however these groups are currently all treated in the same manner, though their responses are quite variable. OvCaRe is working to determine what is responsible for division between ovarian cancers subtypes and developing subtype specific treatments.
OvCaRe is funded through generous donations to the VGH & UBC Hospital Foundation and BC Cancer Foundation. The OvCaRe team is considered a leader in ovarian cancer research, breaking new ground to improve the identification, understanding, and treatment of this disease.
About the British Columbia (BC) Cancer Agency
The BC Cancer Agency provides a comprehensive province-wide, population-based cancer control program for the people of British Columbia, Canada, including prevention, screening and early detection programs, translational research and education, and care and treatment.
The BC Cancer Agency’s mandate covers the spectrum of cancer care, from prevention and screening, to diagnosis, treatment, and rehabilitation. The BC Cancer Agency’s mandate is driven by a three-fold mission: (1) reduce the incidence of cancer, (2) reduce the mortality rate of people with cancer, and (3) improve the quality of life of people living with cancer. This mission includes providing screening, diagnosis and care, setting treatment standards, and conducting research into causes of, and cures for, cancer.
The BC Cancer Agency operates five regional cancer centres, providing assessment and diagnostic services, chemotherapy, radiation therapy, and supportive care. Each of the BC Cancer Agency’s centres delivers cancer treatment based on provincial standards and guidelines established by the Agency.
Research is an essential part of the BC Cancer Agency’s mission to not only find the causes of cancer, but to find better treatments for prolonged life and better quality of life. With direct links between the BC Cancer Agency’s physicians and researchers at its five centres (including the Deeley Research Centre (located in Victoria) and the BC Cancer Agency’s Research Centre (located in Vancouver)), the BC Cancer Agency can quickly translate new discoveries into clinical applications. The BC Cancer Agency’s Research Centre includes eight specialty laboratories including the Michael Smith Genome Sciences Centre, and the Terry Fox Laboratory.
The BC Cancer Agency includes the following among its many accomplishments:
- Canada’s largest fully integrated cancer and research treatment organization;
- the best cancer incidence and survival rates in Canada as a result of the unique and longstanding population-based cancer control system;
- leadership in cancer control with world-renowned programs in lymphoid, lung, breast, ovarian and oral cancer research and care; and
- a unique set of research platforms that form the basis of research and care, including one of the world’s top four genome sciences centres.
About the Vancouver General Hospital (VGH)
The Vancouver General Hospital (VGH) is a 955 bed hospital that offers specialized services to residents in Vancouver and across the province. VGH is also a teaching hospital, affiliated with the University of British Columbia and home to one of the largest research institutes in Canada.
About the British Columbia (BC) Cancer Foundation
The BC Cancer Foundation is an independent charitable organization that raises funds to support breakthrough cancer research and care at the BC Cancer Agency.
Over 70 years ago, the BC Cancer Foundation, led by a group of prominent BC citizens, created what is today the BC Cancer Agency. The Foundation has offices in all five of the BC Cancer’s Agency’s treatment centres – Abbotsford, Fraser Valley, Southern Interior, Vancouver Island and Vancouver.
About the Vancouver General Hospital (VGH) & University of British Columbia (UBC) Hospital Foundation
The VGH & UBC Hospital Foundation is a registered charity that raises funding for the latest, most sophisticated medical equipment, world-class research and improvements to patient care for VGH, UBC Hospital, GF Strong Rehab Centre and Vancouver Coastal Health Research Institute. For more than 25 years, the Foundation and its donors have been a bridge between the essential health care governments provide and the most advanced health care possible.